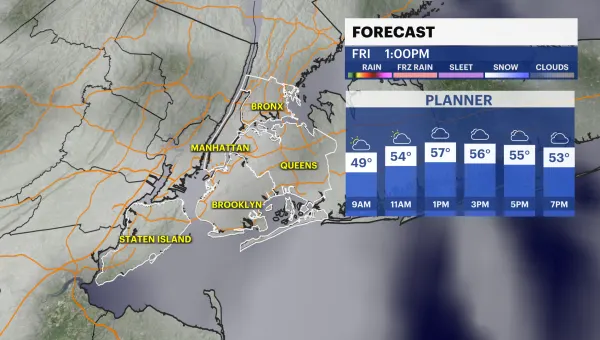
FDA decision expected to generate jobs in Rockland
A decision by the FDA is expected to generate new jobs at a Rockland County biotech firm. For the past two years, Protein Sciences has been making a flu vaccine with no additives in Connecticut. The
News 12 Staff
•
Jun 15, 2015, 7:59 PM
•
Updated 3,230 days ago
Share:

A decision by the FDA is expected to generate new jobs at a Rockland County biotech firm.
For the past two years, Protein Sciences has been making a flu vaccine with no additives in Connecticut. The FDA recently gave the company permission to make that vaccine in Pearl River.
The vaccine, which is being marketed at FluBlok, is different from traditional flu vaccines. Company officials say it doesn't use eggs or preservatives, which can cause reactions in some people.
Previously, the vaccine was produced in a company located in Connecticut. With the FDA's licensing of the lab in Pearl River, an additional 40 people will be added to the 30 already working there.
The FDA is letting Protein Sciences have the exclusive right to make the vaccine until 2025.